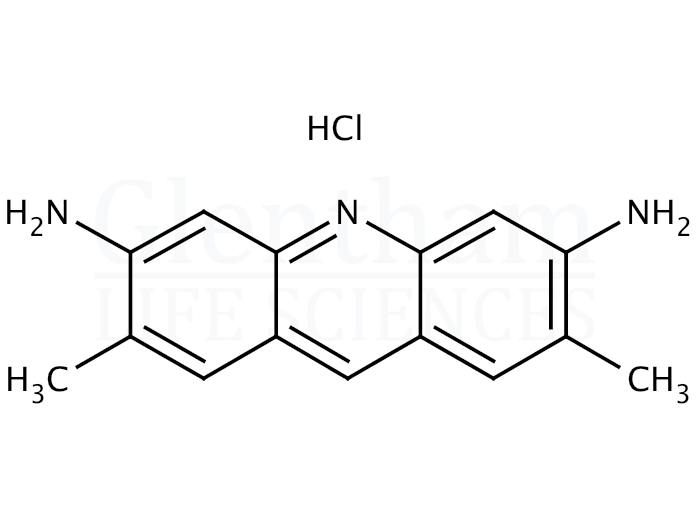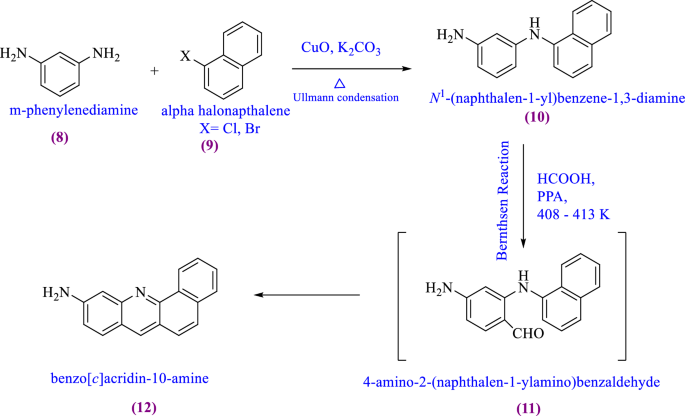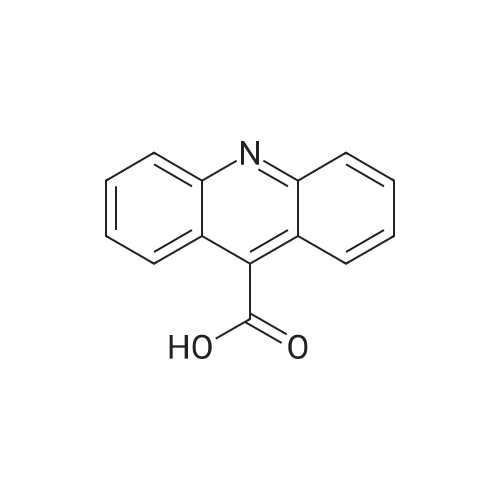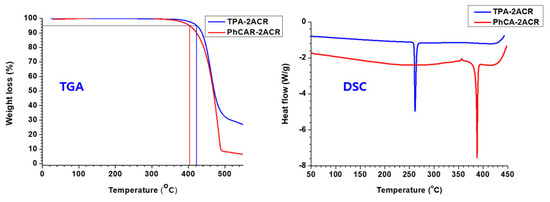
Molecules | Free Full-Text | Acridine Based Small Molecular Hole Transport Type Materials for Phosphorescent OLED Application

Dimethyltin(IV) and palladium(II) complexes derived from 2-benzoylpyridine N(4)-cyclohexylthiosemicarbazone: Synthesis, crystal structures and biological evaluation - ScienceDirect
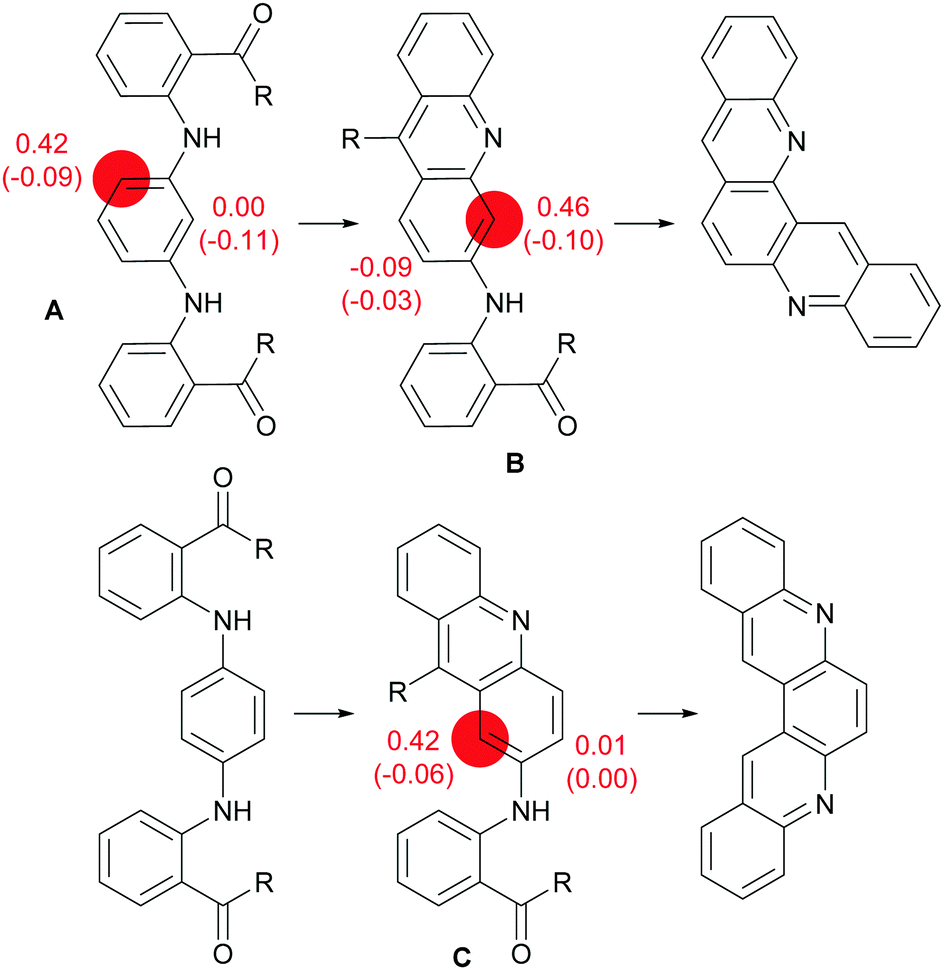
Aza-aromatic polycycles based on triphenylene and acridine or acridone: synthesis and properties - New Journal of Chemistry (RSC Publishing) DOI:10.1039/D1NJ02630E

US20220169607A1 - Carbazole and acridine photoredox catalysts for small molecule and macromolecular transformations - Google Patents
![Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2666086523000024-gr1.jpg)
Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect

China Trans-Dibromobis(triphenylphosphine)palladium(II) CAS No.: 22180-53-6 Manufacturers - Free Sample - Alfa Chemical

Acridine as Bioinspired Corrosion Inhibitors - Bhati - 2023 - Macromolecular Symposia - Wiley Online Library

Alkyl(quinolin-8-yl)phosphine Oxides as Hemilabile Preligands for Palladium-Catalyzed Reactions | Organometallics
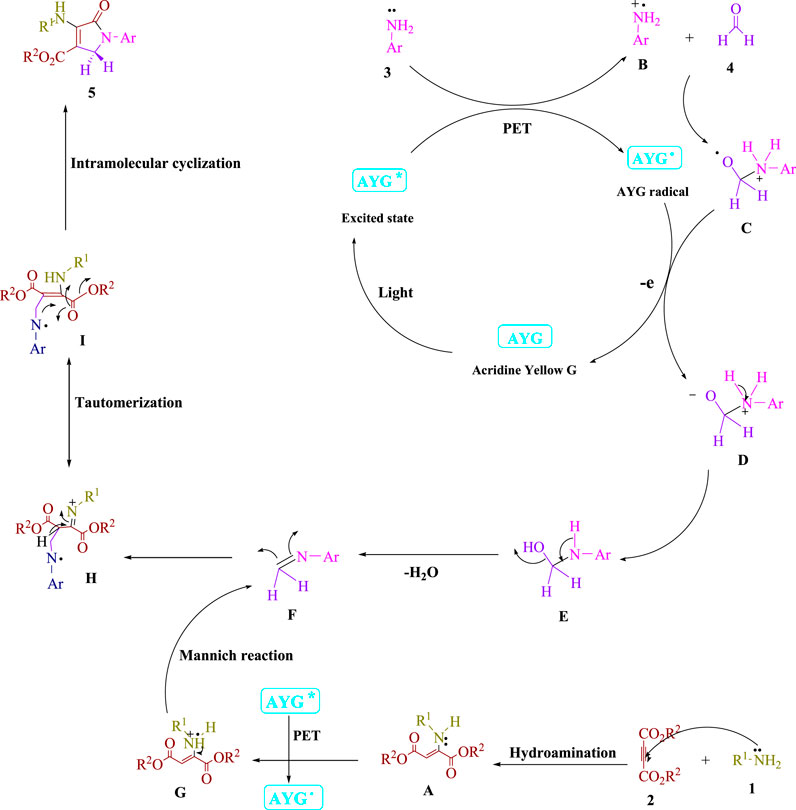
Frontiers | Acridine yellow G (AYG) as a photo-induced electron transfer (PET) photocatalyst employed for the radical Michael–Mannich cyclocondensation of imines

Fluorescent images of acridine orange/ ethidium bromide dual stained... | Download Scientific Diagram

A new synthesis strategy for acridine derivatives to constructing novel host for phosphorescent organic light-emitting diodes - ScienceDirect
![Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2666086523000024-sc2.jpg)
Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect
![Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2666086523000024-gr2.jpg)
Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect

Tripodal N-Heterocyclic Carbene Complexes of Palladium and Copper: Syntheses, Characterization, and Catalytic Activity | Organometallics

Shining Visible Light on Reductive Elimination: Acridine–Pd-Catalyzed Cross-Coupling of Aryl Halides with Carboxylic Acids | Journal of the American Chemical Society
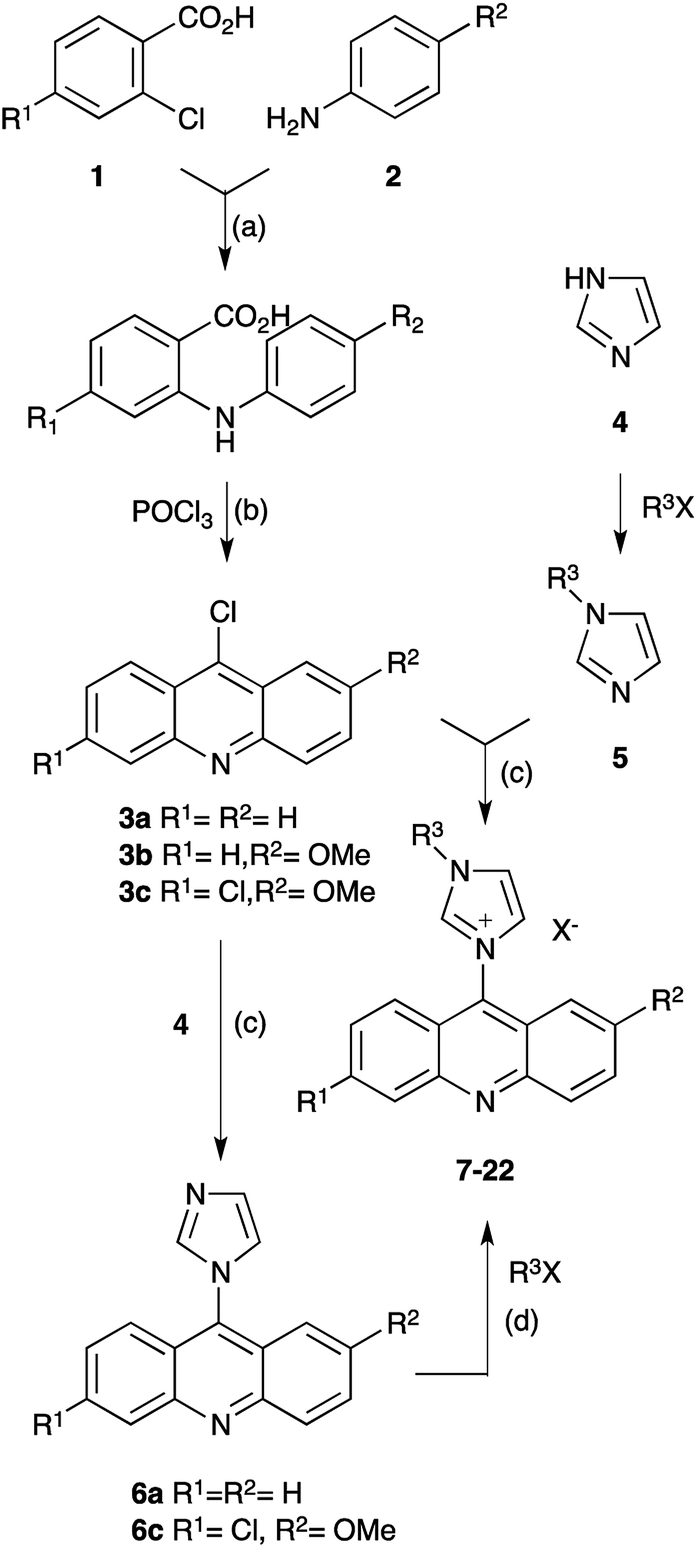
Synthesis and biological study of acridine-based imidazolium salts - RSC Advances (RSC Publishing) DOI:10.1039/C8RA08138G

Palladium Nanocapsules for Photothermal Therapy in the Near-Infrared II Biological Window | ACS Applied Materials & Interfaces
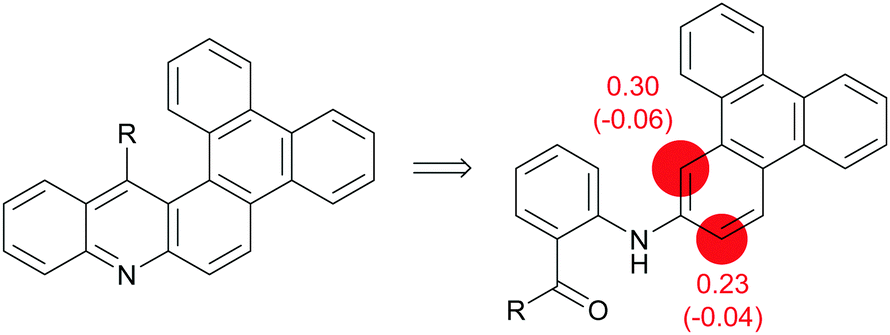
Aza-aromatic polycycles based on triphenylene and acridine or acridone: synthesis and properties - New Journal of Chemistry (RSC Publishing) DOI:10.1039/D1NJ02630E

Synthesis and Spectrophotometric Studies of 9‐Substituted‐4,5‐dimethoxyacridine Multifunctionalizable Fluorescent Dyes and Their Macrocyclic Derivatives - Golcs - 2021 - European Journal of Organic Chemistry - Wiley Online Library
![Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2666086523000024-fx3b.jpg)
Acridine yellow G as a photo-induced electron transfer catalyzed radical metal-free synthesis of tetrahydrobenzo[b]pyran scaffolds in an aqueous media - ScienceDirect
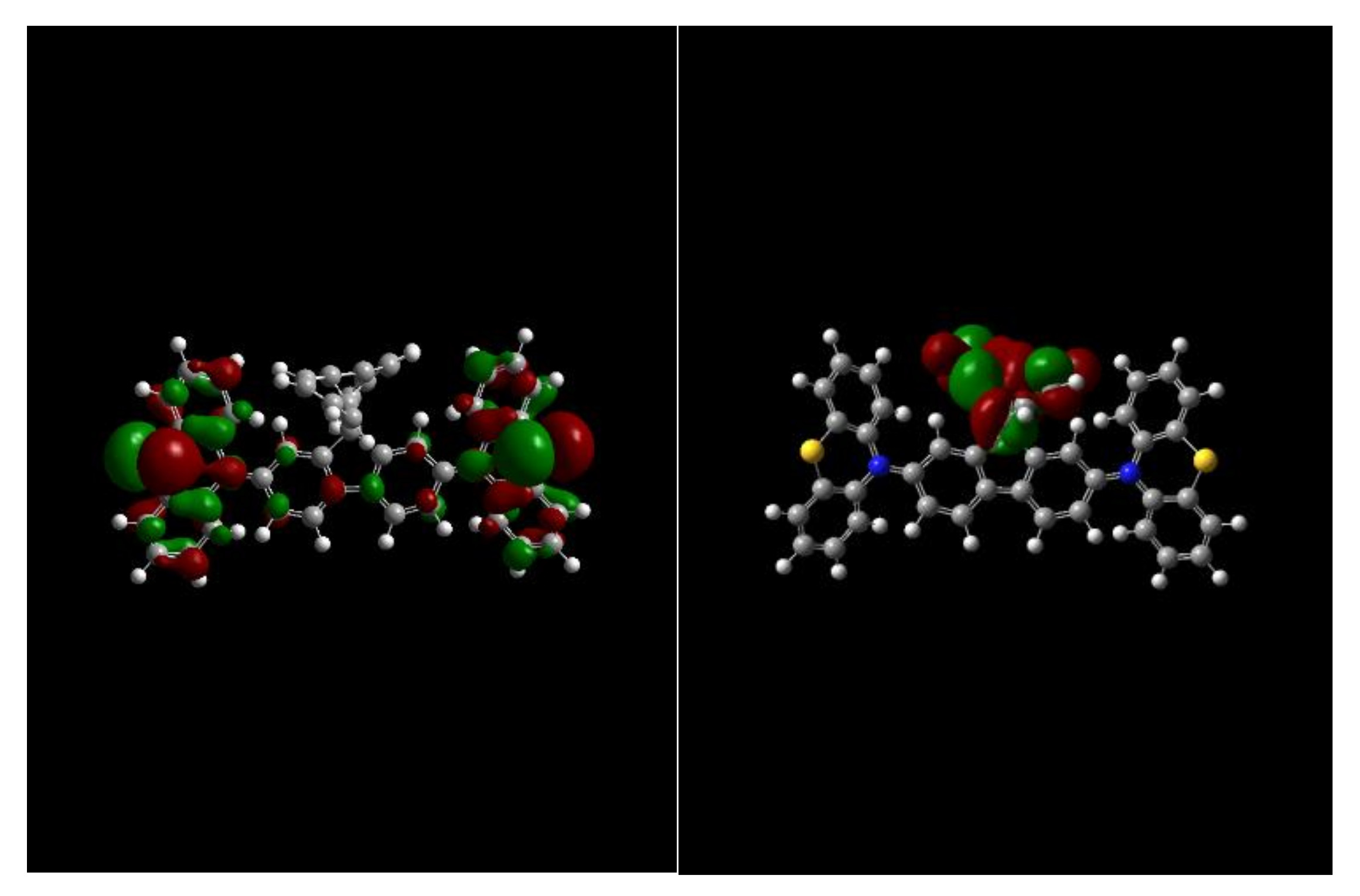
Molecules | Free Full-Text | Utilizing a Spiro Core with Acridine- and Phenothiazine-Based New Hole Transporting Materials for Highly Efficient Green Phosphorescent Organic Light-Emitting Diodes

Palladium-Catalyzed Annulation of Phenazastannines with 9-(Dibromomethylene)fluorene and -(thio)xanthenes: Facile Synthesis of Acridine Moiety-Containing Bis(tricyclic) Aromatic Enes | Organic Process Research & Development
![Molecules | Free Full-Text | Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line Molecules | Free Full-Text | Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line](https://www.mdpi.com/molecules/molecules-25-05862/article_deploy/html/images/molecules-25-05862-g001.png)