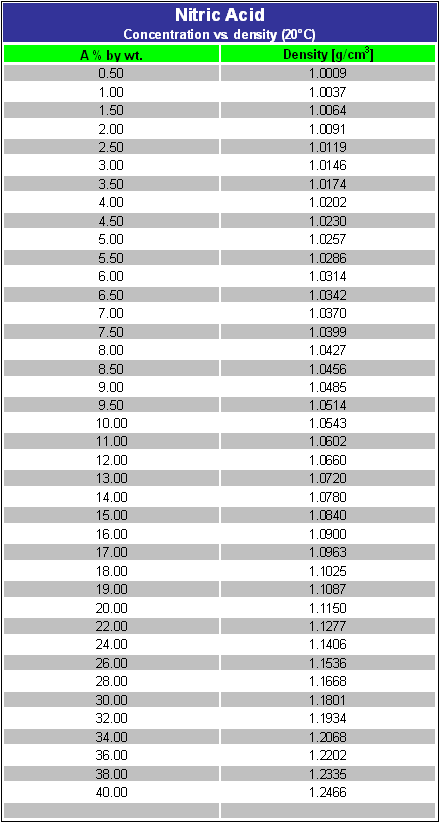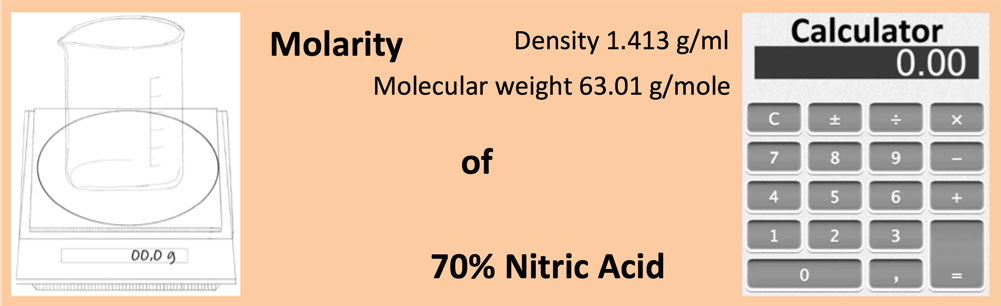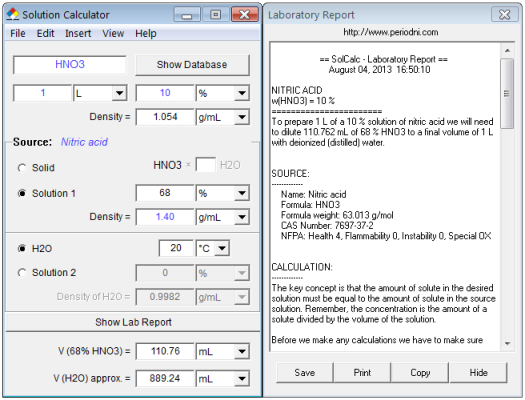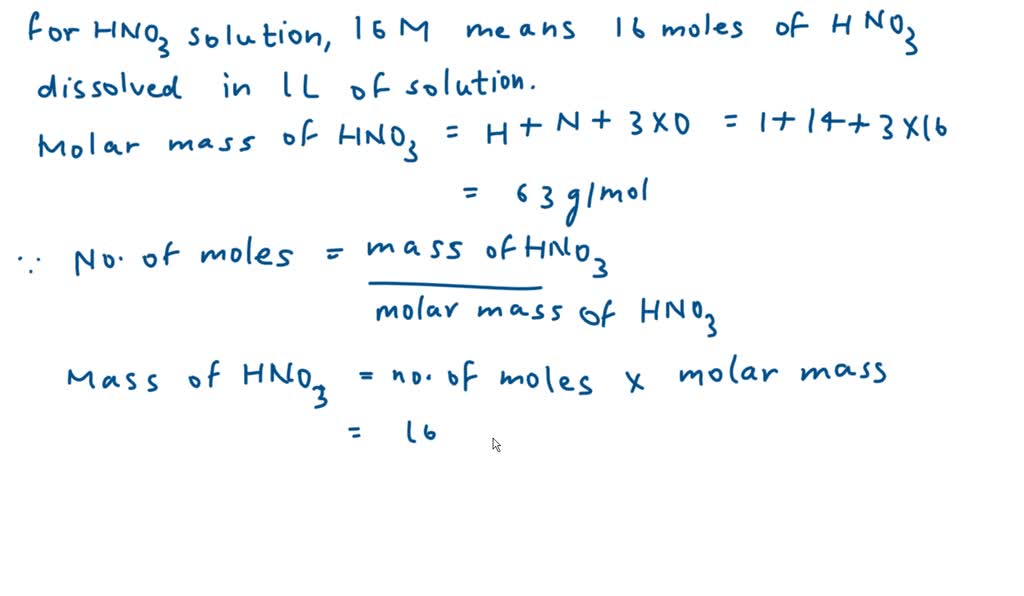
SOLVED: Commercial nitric acid is 16 M HNO3 and it has density of 1.42 g/mL Calculate the masspercent of HNOz in commercial nitric acid?
A certain solution contains 16.9 g of HNO3 dissolved in 125 mL of a substantive solution. Water is added until the volume is 175 mL. What is the molarity of Hno3 in

SOLVED: Enter your answer in the provided box: Nitric acid (HNO;) is used in the production of fertilizer; dyes, drugs; and explosives Calculate the pH of # HNOz solution baving : hydrogen

Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g //mL and the mass percent of nitric acid in it being 69%.

Calculate the concentration of nitric acid in moles per litre in a sample which has density 1.41g/mL - YouTube

Calculate the concentration of nitric acid in moles per litre in a sample which has a density - YouTube

Question Video: Calculating the Concentration of Nitric Acid via Titrating against a Known Volume of Potassium Hydroxide | Nagwa



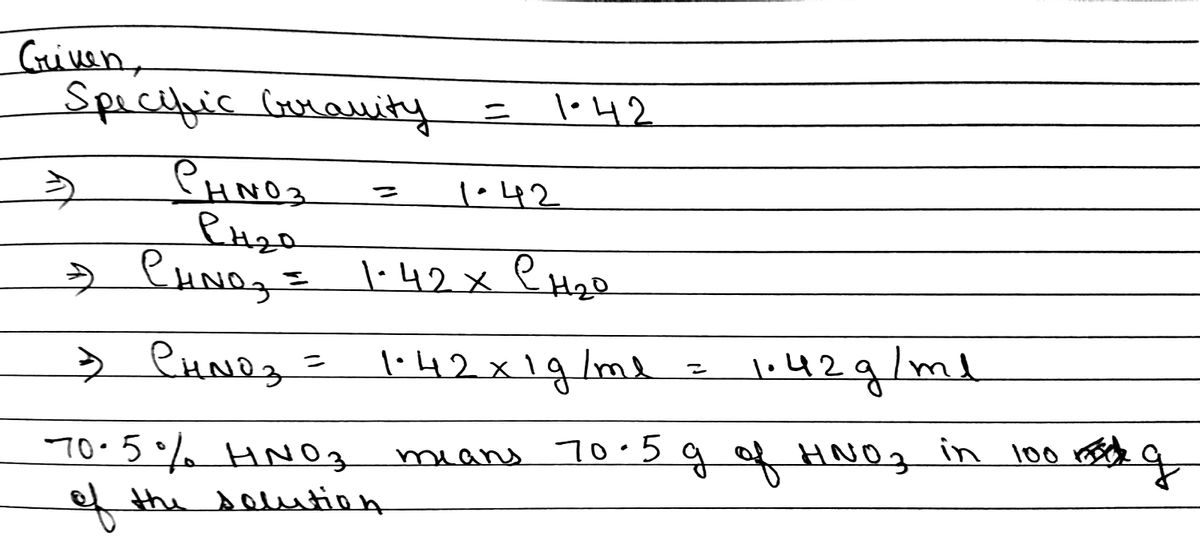
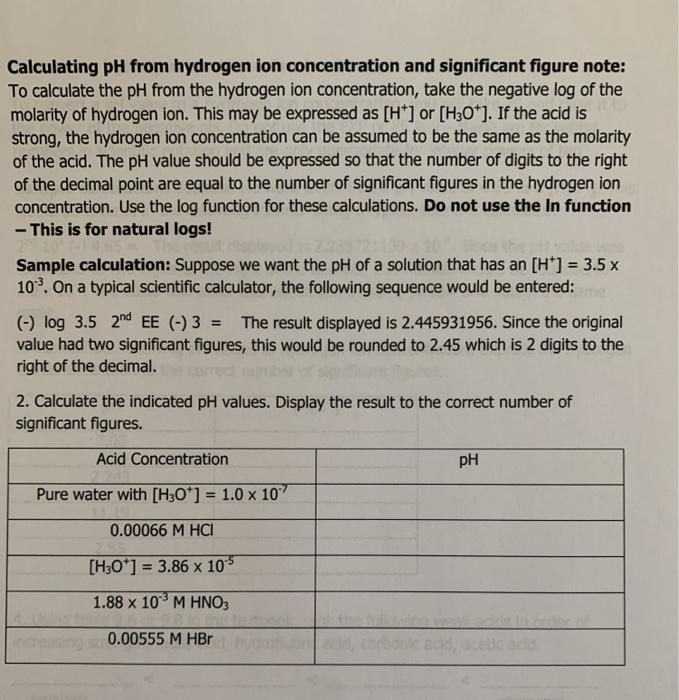


![Solved For a 0.12 M solution of HNO3, calculate: [+], pH, | Chegg.com Solved For a 0.12 M solution of HNO3, calculate: [+], pH, | Chegg.com](https://media.cheggcdn.com/media/6a7/6a7384ad-d27d-4f6c-ba66-edb5228d98b9/php7isMK0.png)