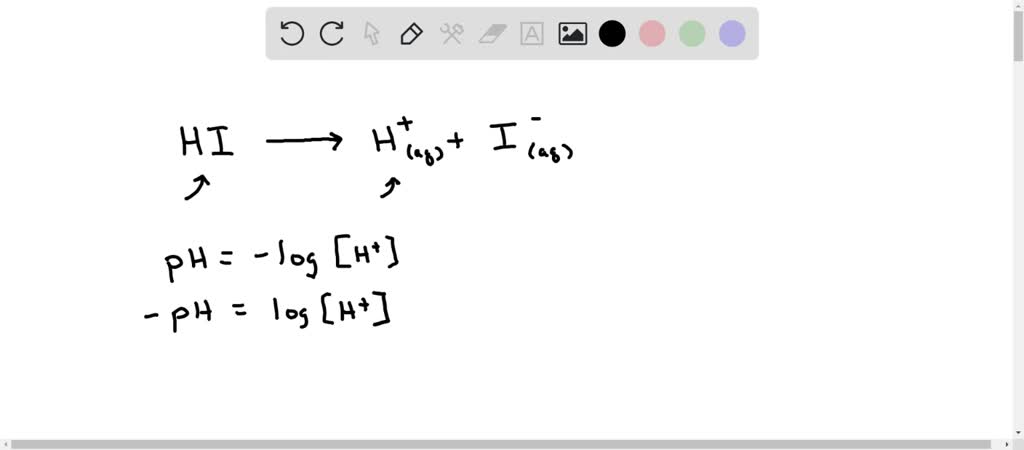
SOLVED: Calculate the concentration of an aqueous HI solution that has pH=2.50 . HI is a strong acid.
![SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice, SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice,](https://cdn.numerade.com/previews/c92ef7f1-c83f-4027-9f05-6b11ebe1b21e_large.jpg)
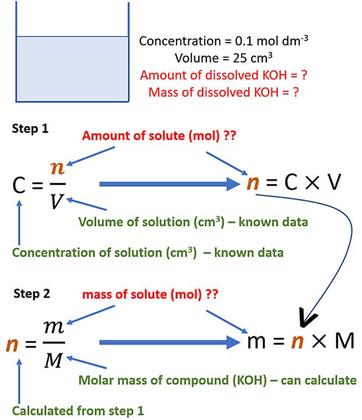
SOLVED:MATHEMATICAL Calculate the hydrogen ion concentration, [H^+] for each of the following materials: (a) Saliva, pH 6.5 (b) Intracellular fluid of liver, pH 6.9 (c) Tomato juice, pH 4.3 (d) Grapefruit juice,

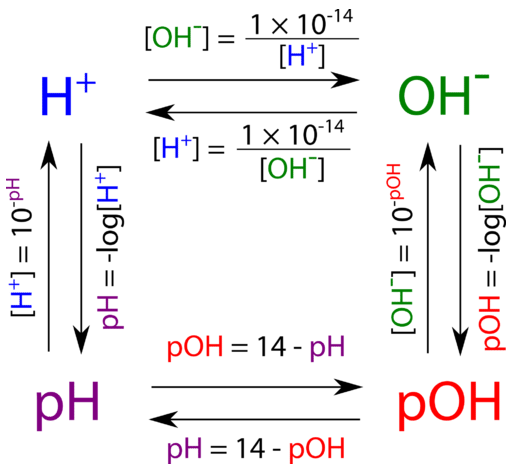
Calculating Hydronium ion concentration from pH (and identifying molecules based on pH) : r/chemhelp

How to Calculate ph if Hydrogen ion Concentration is Given For All Stud... | Concentration, Student, Calculator




![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)

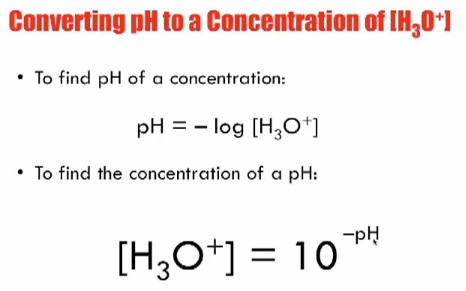
:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)

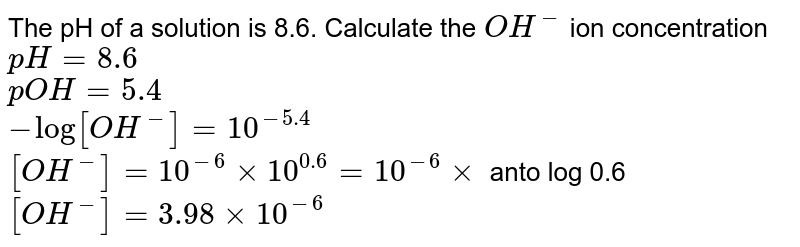



![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)
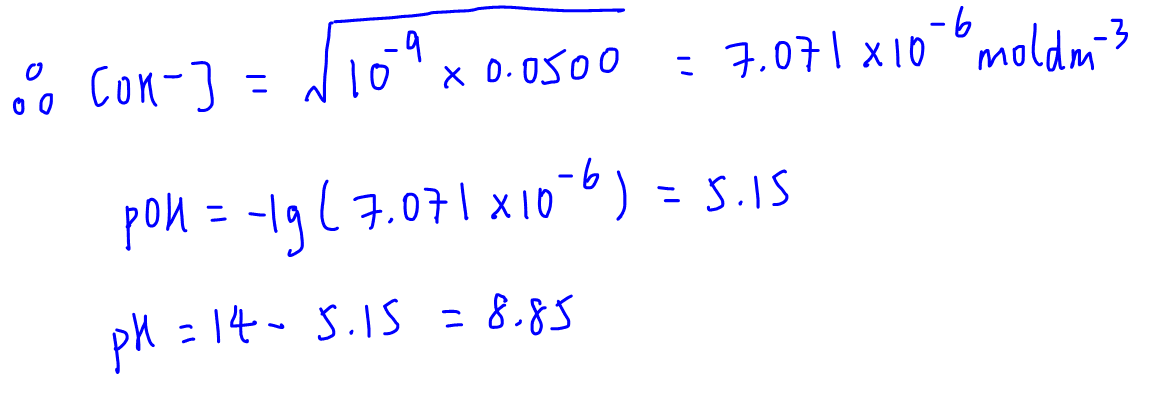

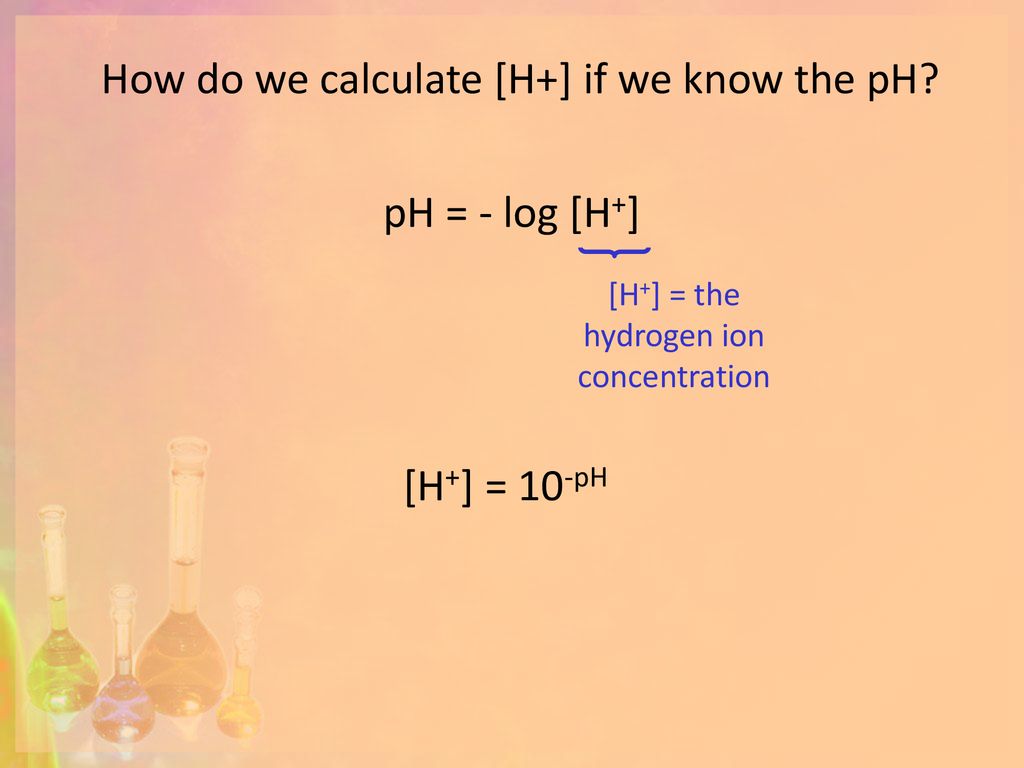

![Calculating [OH-], pH and pOH from Kb Calculating [OH-], pH and pOH from Kb](https://www.mi.mun.ca/users/pfisher/chemistry1011_135/img007.gif)