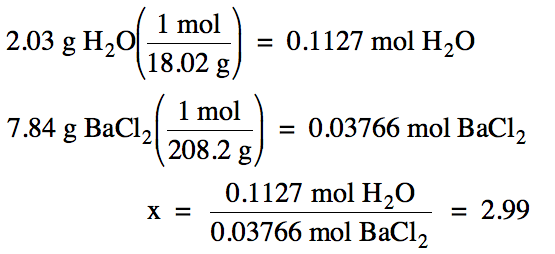Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? - Video & Lesson Transcript | Study.com

Question Video: Determining the Water of Hydration of Magnesium Sulfate Hydrate Given the Number of Moles of Water Liberated | Nagwa

Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? - Video & Lesson Transcript | Study.com
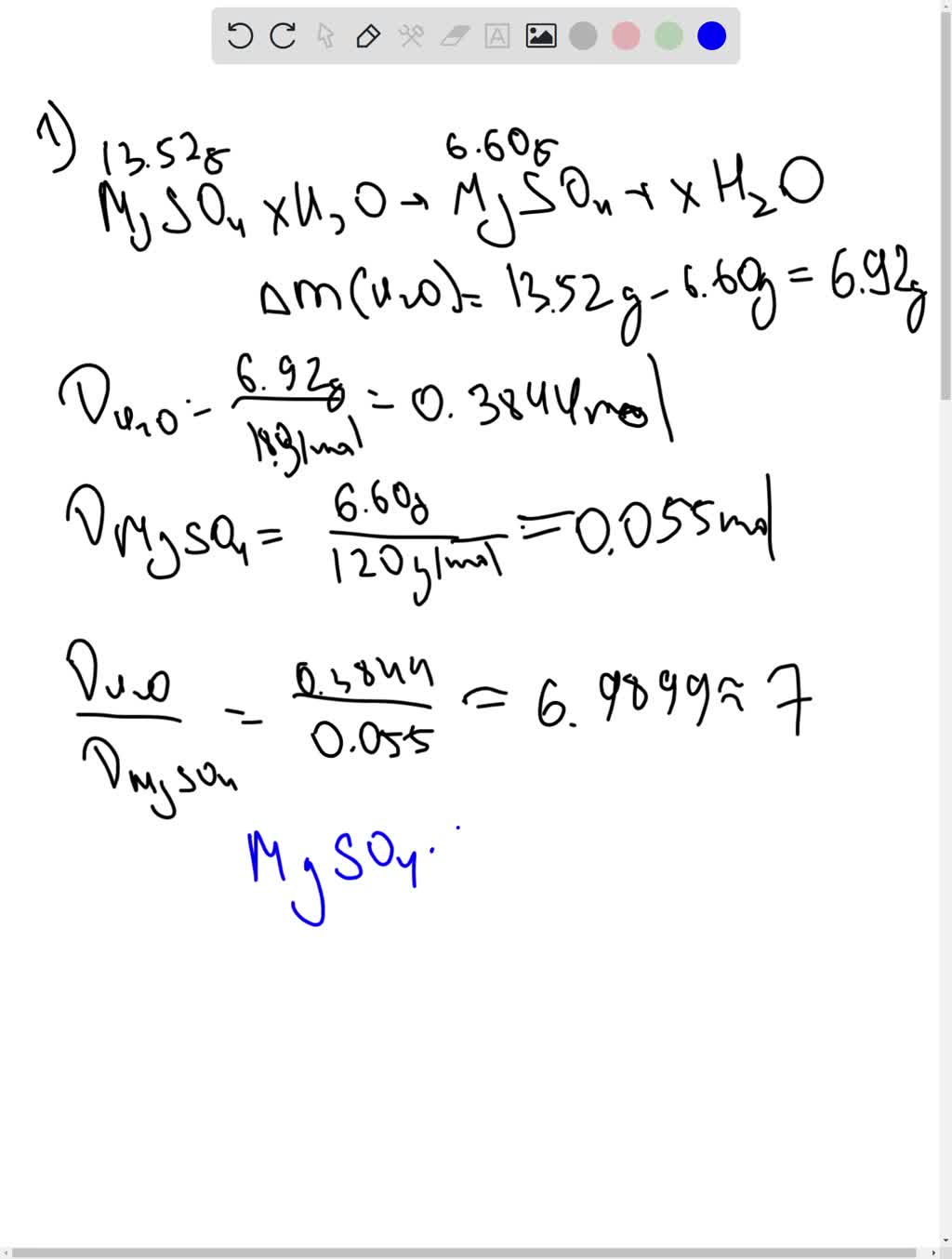
SOLVED: Use the magnesium chloride hydrate unknown, in the Empirical formula of copper oxide lab, measure out 4.00 g of the unknown magnesium chloride and heat for 5 minutes. a. What is
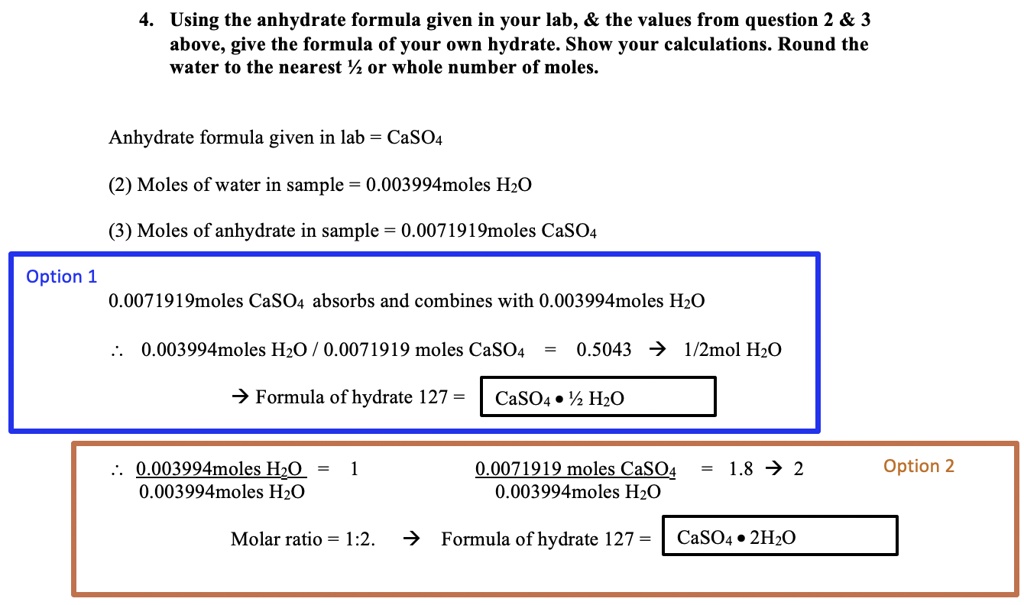
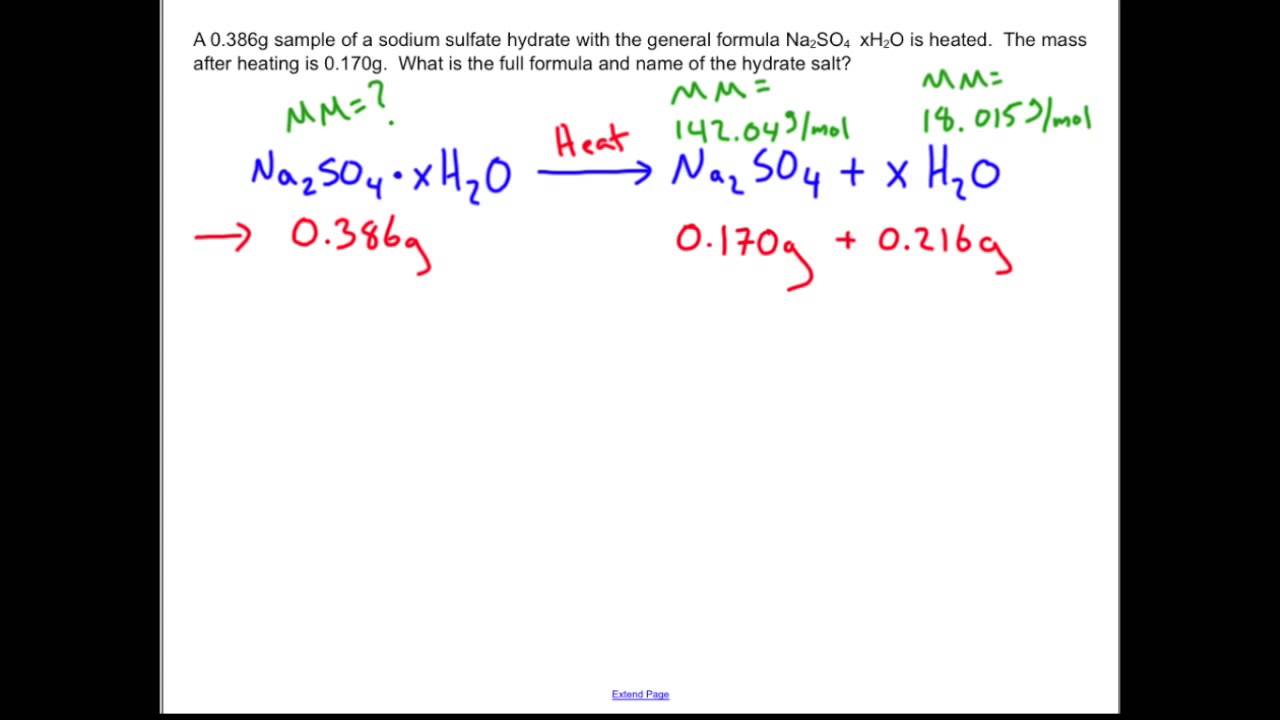
SOLVED: Using the anhydrate formula given in your lab, the values from question 2 3 above, give the formula of your own hydrate. Show your calculations. Round the water to the nearest %/

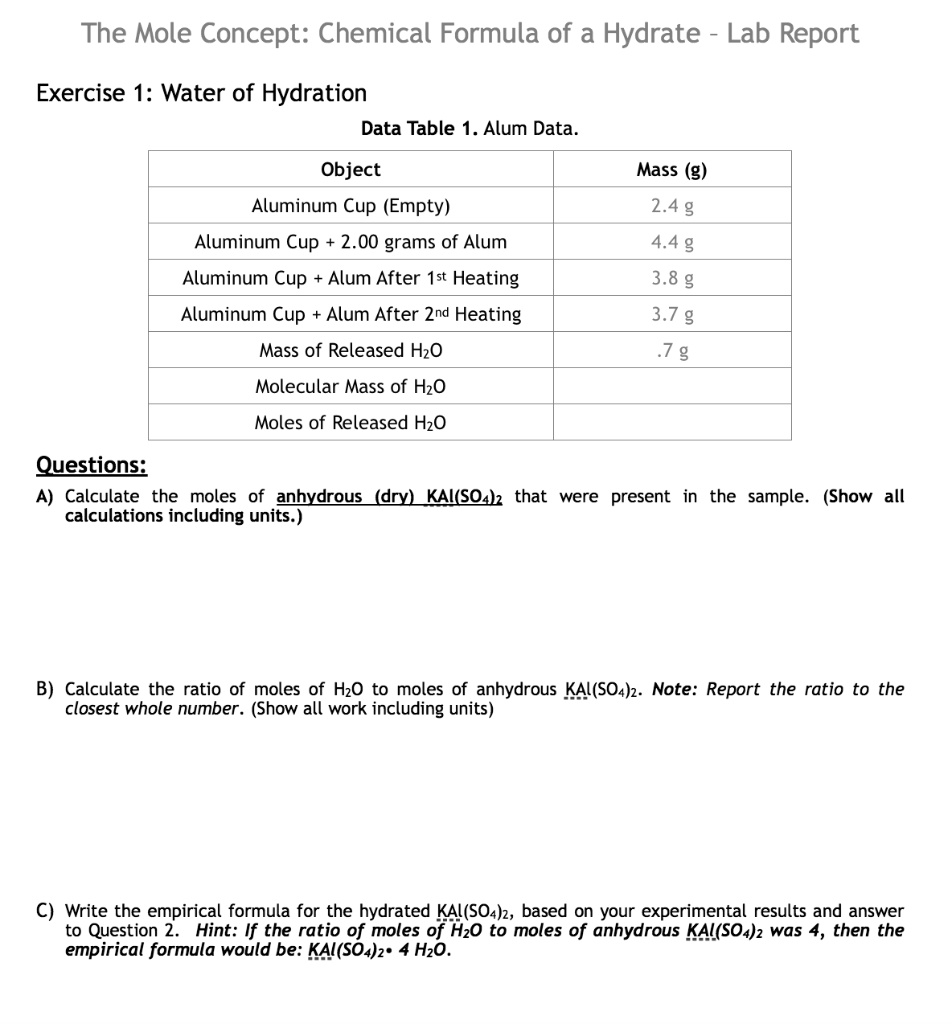
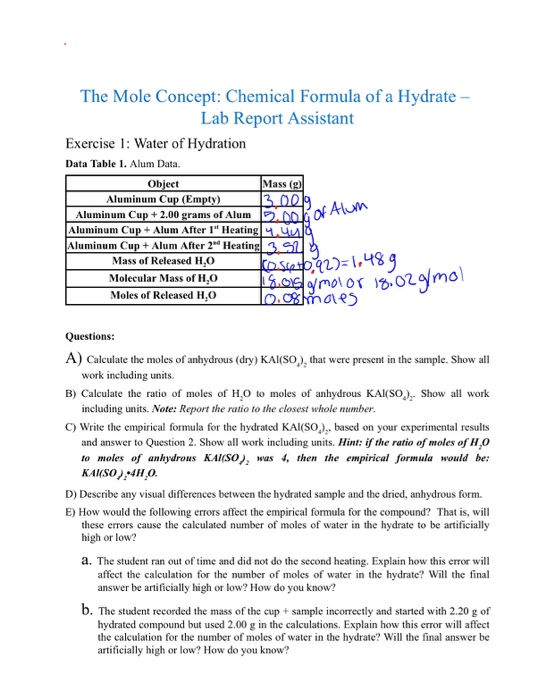
Sample lab report - Experiment # Kayla Morren Chemical Formula of a Hydrate Objective: The objective - Studocu